Case Presentations
Here you will find cases in which physical examination played a key role in making the diagnosis. Try to work through the cases one step at a time and see if you arrive at the correct diagnosis.
![]()
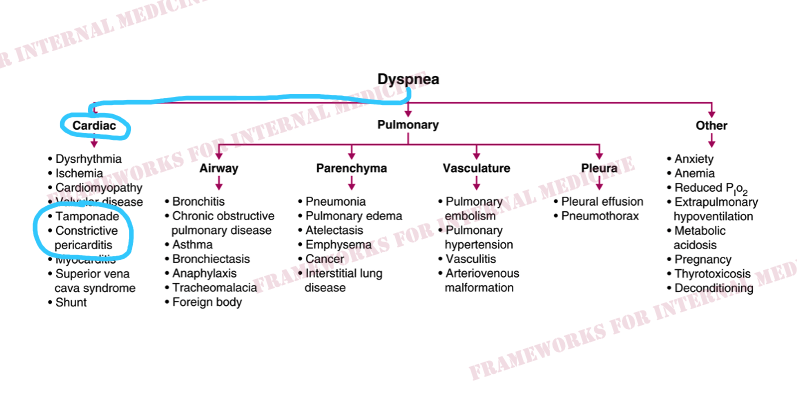
Patient 1: Dyspnea
The presence of de Musset’s sign (to-and-fro head bob) is consistent with your hypothesis. So you look for more evidence.
And you find it in the form of Corrigan’s pulse. You continue to evaluate the peripheral pulses and find more evidence to support your hypothesis.
This should make it easier to appreciate.
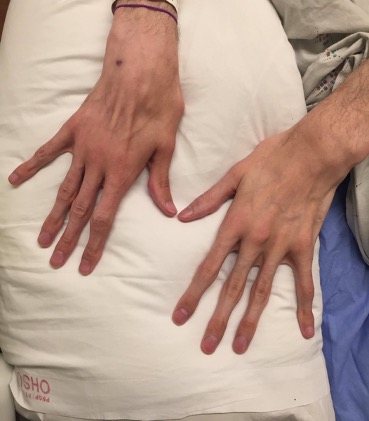
Next you look at the patient’s fingernails. What do you see?
The patient is having trouble seeing what you see in his nail bed. So you use a light source to help him.
You continue to evaluate the peripheral pulses, finally reaching his foot:
Before you even listen to the patient’s heart, your diagnosis has all but been made. And when you listen, you know exactly what to listen for. “The ears can’t hear what the mind doesn’t know”
You have just diagnosed severe aortic insufficiency with your eyes, your ears, and a Q-tip.

Patient 2: Fever
 This should generate a hypothesis.
This should generate a hypothesis.
With your hypothesis in mind, you listen to the patient’s heart. You anticipate what you might hear. “The ears can’t hear what the mind doesn’t know.”
An additional diagnosis has now been made.
Based on the holosystolic murmur at the apex that you anticipated you would hear, you diagnose the patient with mitral valve endocarditis. Two days later, his heart sounds change. Take a listen.
Two days later, you no longer hear the pericardial friction rub. In fact, his heart sounds are difficult to hear at all. He develops hypotension and pre-syncope and his neck looks like this:
This should generate a hypothesis.
You confirm your hypothesis with a bedside maneuver (video features a different patient with the same diagnosis):
You have diagnosed infective endocarditis of the mitral valve with pericardial involvement, evolving to pericardial effusion with cardiac tamponade. All with your eyes and ears.
Patient 3: Weakness
A middle-aged man walks into your clinic complaining of weakness. You take a moment to observe his gait. What do you notice?
This should generate a hypothesis.
You reference your framework for weakness and try to determine the location of the lesion to narrow your differential diagnosis. The patient’s gait has already provided a clue (spasticity).

 So you check his reflexes next.
So you check his reflexes next.
The hyperreflexia suggests an upper motor neuron lesion. Next, you check for Hoffmann’s sign, which if present, would suggest a cervical lesion.
You have just diagnosed cervical myelopathy. Using only your eyes and a reflex hammer.
Patient 4: Progressive dyspnea
A middle-aged woman presents with progressive dyspnea. You start with her hands. What do you notice? Does it generate any hypotheses in your mind?

Could these small erythematous lesions be telangiectasias? Let’s see if they blanch and refill by pressing on one of them.
Indeed, they do, confirming your suspicion. What condition comes to mind? Perhaps you are thinking about hereditary hemorrhagic telangiectasia (HHT). Can it cause dyspnea? Yes, via pulmonary AVMs. What other condition can present with telangiectasias that may involve the lungs?
Your new hypothesis leads you to examine the hands further, discovering that the patient has a hard time completely straightening her fingers. You ask her to make the universal sign of prayer.

You search her skin for other signs of the condition that is now at the forefront of your mind. What is this hard, white nodule on her elbow? Does it further advance your hypothesis?

So what about the dyspnea? How does the condition you have in mind affect the lungs? You look for evidence of pulmonary hypertension on exam. You start with the jugular venous pulse. What finding is present?
Then you auscult the heart. What finding is present? What is your ultimate diagnosis?
This patient has scleroderma with systemic involvement (SSc). Typical cutaneous manifestations include telangiectasias, sclerodactyly (+ Prayer sign), and calcinosis cutis (nodule on her elbow). SSc can cause pulmonary hypertension, leading to Kussmaul’s sign and loud P2.
A standardized test would provide all of these clues on a silver platter. Any machine can synthesize them and make the diagnosis. The clinician must not only synthesize, but gather these clues at the bedside. Your search must be hypothesis-driven or you might miss them.
Anticipation is key in medicine. The eyes can’t see what the mind doesn’t know. The ears can’t hear what the mind doesn’t know.
Patient 5: Extra Heart Sound
So you hear an extra heart sound. Now what? First: Is the extra sound near S1 or S2? Each scenario is associated with a different differential. Listen to this clip. Is the extra sound here near S1 or S2?
Not sure you hear three sounds? To orient you, listen to normal S1 and S2. There are two sounds here.
Back to our patient. So is the extra sound near S1 or near S2?
It is near S2. What is the differential?
DDx: Split S2, S3 gallop, Opening snap, Pericardial knock, Tumor plop
Persistently split S2 and S3 gallop are most common. Let’s start there. How do you distinguish them?
Split S2: best heard over BASE, with DIAPHRAGM, closer to S2.
S3: best heard over APEX, with BELL, further from S2.
Take a listen to this clip. There is an extra sound near S2. What is it? It is heard over the base of the heart with the diaphragm of the scope (see key in top left of video).
This is a persistently split S2. (S1 is also split.)
What about the the opening snap? It occurs further from S2 than the split S2, but closer to S2 than the S3 gallop and pericardial knock. It is best heard with the diaphragm and closer to the apex. Take a listen in this patient with severe mitral stenosis:
What about the the pericardial knock? It occurs outside the range for a split S2 and the OS of mitral stenosis, but not as far from S2 as an S3. It is best heard with the diaphragm over the LLSB/apex. Take a listen in this patient with constrictive pericarditis:
So back to our patient. What is the extra sound? Orienting yourself to the cardiac cycle is critical. Is the sound near S1 or near S2? How far from S2 is the sound? Is it high- or low-pitched? Is it best heard over the base or apex?
Our patient has an S3 gallop.
Never forget importance of history and associated findings. The exam is not practiced in a vacuum. Is the patient from a country where rheumatic heart disease is prevalent? Does the patient have sharp and deep X/Y descents indicative of constriction? Do they have other signs of heart failure?
Patient 6: Transient Heart Sound
You hear an extra transient heart sound near S1. Now what?
Not sure you hear three sounds? Here is normal S1 and S2 to serve as a control.
There are two sounds. Listen to this clip and then re-listen to the above clip. When you do, you will hear three sounds. Two near where S1 should be, followed by S2.
So what’s the differential for extra transient sounds near S1?
DDx: Split S1, S4 gallop, Ejection click
Split S1 and S4 gallop can be challenging to distinguish because both are best heard over the APEX area. However, the split S1 sounds are closer together than the S1-S4 interval. And the S4 is best heard with the BELL of the scope. Listen to this split S1:
Now take a listen to this S4 gallop:
Notice that the S1-S4 interval is longer compared to the split S1 above. And while we are listening over the same area of the chest (apex), the bell is being used rather than the diaphragm.
What about the ejection click? It is perhaps the easiest to distinguish because it is often heard over the BASE of the heart – very atypical for the split S1 and S4. The click is best appreciated with the diaphragm of the scope as it is higher pitched.
So back to our patient. What is the extra sound?
It is heard over the base of the heart with the diaphragm. This is an ejection click. (It was picked up on routine exam and led to the diagnosis of a severely dilated aortic root.)
Remember that the exam is never performed in a vacuum. You will also have the benefit of the history and other findings. Does the patient have longstanding HTN (S4)? Does the patient have a giant a wave with an RV heave suggestive of pulmonary hypertension (click)?
Patient 7: Exertional Dyspnea
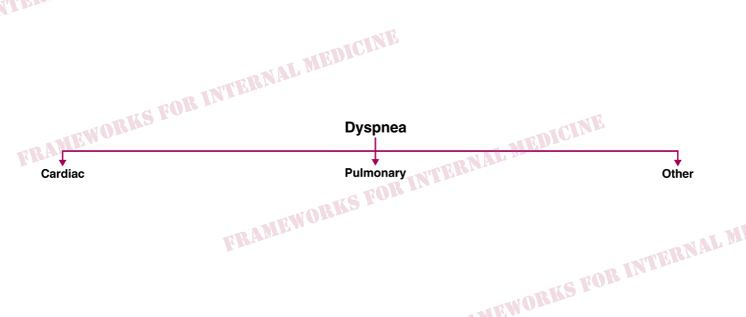
A 50-y/o man presents with exertional dyspnea. The two main systems responsible for dyspnea are the heart and the lungs.
 The jugular venous pulse can serve as a pivot point. It can take you toward or away from the heart. With this in mind, you evaluate the patient’s neck. What do you notice?
The jugular venous pulse can serve as a pivot point. It can take you toward or away from the heart. With this in mind, you evaluate the patient’s neck. What do you notice?
Not only is the JVP elevated, but it appears to rise with inspiration. This is known as Kussmaul’s sign.
In the above video, you may have also noticed a mark on the patient’s skin, just below the “a wave” in our logo. Here is the mark up close:

What is it?
You ask the patient when he had radiation therapy to his chest, and he gives you a surprised look. “How did you know?”
“I haven’t thought about it in 30 years. I had Hodgkin’s lymphoma when I was 17 years old. They shot radiation at my chest. Why does it matter, doc?”
Next, you listen to the patient’s heart, anticipating what you might hear. “The ears can’t hear what the mind doesn’t know”.
You hear an extra transient sound near S2. Your differential is split S2, S3 gallop, opening snap, and pericardial knock. The location, pitch, distance from S2, and the associated history and JVP findings make this sound most likely to be a pericardial knock.
You diagnose this patient with radiation-induced constrictive pericarditis. With your eyes and ears. The effects of radiation therapy can show up decades later, when patients have all but forgotten they even had it.
Patient 8: Heart Failure
A man presents to you with the clinical syndrome of heart failure (weight gain, orthopnea, elevated JVP, etc.). BP is 144/48 mm Hg.
This should generate a hypothesis. Yoo you look for other specific physical findings. What do you notice in this video?
Quincke’s pulse is consistent with your hypothesis, so you look for more evidence in his neck. And you have found it.
You then listen to the patient’s heart in anticipation of hearing a decrescendo diastolic murmur and confirming your suspicions.
But there is no decrescendo diastolic murmur. Further, his echocardiogram is totally normal. There is no aortic valve pathology. There is no systolic dysfunction.
Wide pulse pressure, Quincke’s pulse, and Corrigan’s pulse are not specific for aortic regurgitation. They are physical manifestations of a hyperkinetic state from ANY cause.
The combination of high-output physiology and HF should generate a new hypothesis. You test your hypothesis with right heart catheterization.

You have diagnosed the patient with high-output heart failure. What’s causing it?
Retrospective history reveals the consumption of 3-4 glasses of wine daily. Hypothesis-driven laboratory investigation is pursued.

You have now diagnosed the patient with high-output heart failure secondary to thiamine deficiency (wet beriberi).
Alcohol leads to thiamine deficiency through a variety of mechanisms, including poor diet and decreased absorption of thiamine in the GI tract.
Why is the diagnosis of wet beriberi so critical to make? It is curable. Take a look at BP and PP over time with alcohol cessation and thiamine replacement:

The risk here would have been to diagnose the patient with heart failure with preserved systolic function and then call it a day. “This is a very common diagnosis, take these diuretics and you’ll feel better”.
The observation of the physical findings changed everything.
This patient was cured without need for diuretics or other long-term meds.
The observation of wide pulse pressure and other signs of high-output physiology can provide a pivotal clue to the diagnosis of high-output heart failure, particularly when echo is uninformative.
Patient 9: Rapid Weight Gain
A young man comes to your clinic for evaluation of rapid weight gain. He has heard “diet and exercise” several times before he sees you.

The driver license photo was taken ~9 months prior.
You make some observations, leading you to generate a hypothesis.
Based on your hypothesis, you examine the patient further. And you make several more important observations, increasing the likelihood of your hypothesis.



You remember that skin thickness can be an important sign in this condition, from Lynn Loriaux’s 2017 @NEJM review.
(Examiner’s hand is shown above, patient’s below.)
You now have a clinical syndrome that is consistent with Cushing’s syndrome.
A confirmatory test is your next step.
Urine free cortisol (confirmatory test): 7,960 mcg/24H (!)

You have now confirmed the diagnosis of Cushing’s syndrome. The next question is, is it ACTH-dependent or ACTH-independent?
A plasma ACTH level is necessary to make this determination.
Plasma ACTH level is 496 pg/mL (normal <50)
Where is that ACTH coming from? The pituitary gland (ectopic) or elsewhere (ectopic)?
To determine this, inferior petrosal sinus sampling is necessary.
IPSS shows a ratio <3, confirming an ectopic source of ACTH.


Chest imaging ultimately reveals the presence of a bronchial carcinoid tumor.

You have diagnosed ACTH-dependent Cushing’s syndrome from an ectopic source using only your eyes and hypothesis-driven laboratory and imaging tests.
Patient 10: Ascites
You are rotating on the Procedure Service and your team is asked to perform a routine “therapeutic” paracentesis on a patient with cancer. You walk into the room to meet the patient and this is what you see. This finding should generate a hypothesis.
A “diagnostic” paracentesis wasn’t requested, but the underlying cause of ascites in this case has never been questioned. You consult your framework for ascites: The first question you want to know is whether the process is driven by portal hypertension or not.
Serum albumin is 3.1 g/dL and ascitic fluid album is 0.8 g/dL, yielding a serum-ascites albumin gradient (SAAG) of 2.3 (>1.1), which is consistent with a portal hypertensive process. Next you wonder if the cause of portal HTN is prehepatic, hepatic, or posthepatic. For this you return to the cornerstone of diagnostic medicine, the history and physical examination. You recall the hypothesis generated by the dilated forehead veins and you evaluate the JVP.
Next you wonder if the cause of portal HTN is prehepatic, hepatic, or posthepatic. For this you return to the cornerstone of diagnostic medicine, the history and physical examination. You recall the hypothesis generated by the dilated forehead veins and you evaluate the JVP.
The patient is upright in the video. What do you think right atrial pressure is? High? Low? Normal?
It is markedly elevated. Pointing you in the direction of posthepatic causes of portal hypertension.



Patient 11: Jaundice


Patient 12: Hypoxemia
A young woman presents with progressive dyspnea. You walk into the room and this is what you see. What finding is present?


 Next we revisit the physical examination for more clues. There is profound peripheral cyanosis. But what else do you notice? If you don’t specifically look for this finding you might never notice it. “The eyes can’t see what the mind doesn’t know.”
Next we revisit the physical examination for more clues. There is profound peripheral cyanosis. But what else do you notice? If you don’t specifically look for this finding you might never notice it. “The eyes can’t see what the mind doesn’t know.”


Patient 13: Discordant Heart Failure
A 60 y/o man w chronic systolic HF is admitted with increasing dyspnea on exertion, orthopnea, and fatigue. Says his weight hasn’t changed much recently. We perform a quantitative assessment of the jugular venous pulse. Video starts w patient at 60 degrees and then reclines.
The pulse is visible just above the clavicle at 60 degrees. We estimate RA pressure to be mildly elevated at ~12-14 cm H2O (9-10 mm Hg). Are you surprised? Do symptoms seem out of proportion to RA pressure? Next we perform a qualitative assessment. What do you notice?
So right-sided pressures are only mildly elevated. What about the left? How can we tell? Abdominojugular reflux. A sustained rise in JVP >3 cm for more than 10-15 sec while abd pressure is continuously applied is highly suggestive of elevated wedge pressure >15 mm Hg.
Based on the patient’s history and our exam, we predict mildly elevated RA pressure with markedly elevated wedge pressure. Let’s see what right heart catheterization shows.

 Why is this interesting? Typically R- and L-sided pressures are concordant in patients with L-sided HF. In this case, there is discordance. Wedge pressure >> RA pressure. This occurs in a minority of HF patients.
Why is this interesting? Typically R- and L-sided pressures are concordant in patients with L-sided HF. In this case, there is discordance. Wedge pressure >> RA pressure. This occurs in a minority of HF patients.



Patient 14: Migratory Arthritis
 20K white blood cells but no organisms. She was taken for a washout procedure for presumed septic arthritis. And when the knee didn’t improve, she was taken for another one. And when she still didn’t improve, she was transferred to our hospital
20K white blood cells but no organisms. She was taken for a washout procedure for presumed septic arthritis. And when the knee didn’t improve, she was taken for another one. And when she still didn’t improve, she was transferred to our hospital
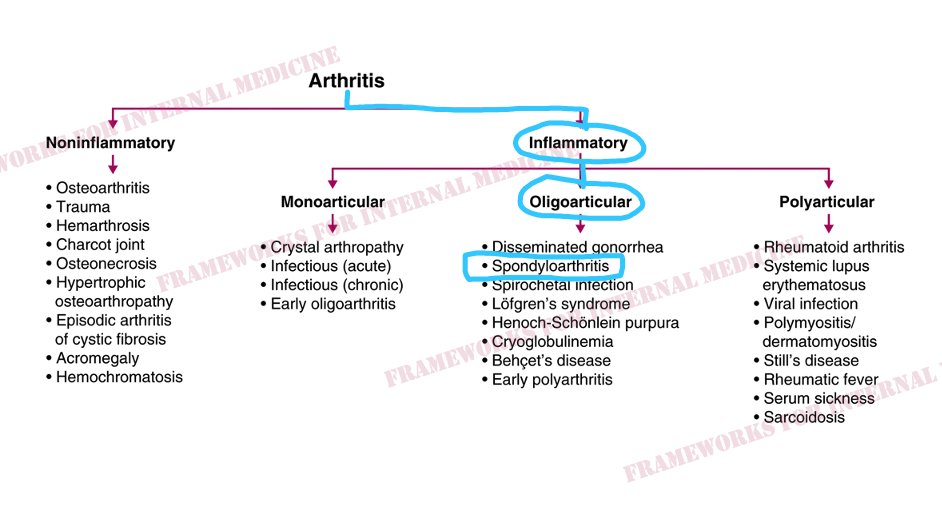
We consult our framework for arthritis.
 Inflammatory arthritis is suggested by the cardinal physical findings of inflammation: erythema (rubor), warmth (calor), pain (dolor), and swelling (tumor). As seen in this elbow joint from another patient:
Inflammatory arthritis is suggested by the cardinal physical findings of inflammation: erythema (rubor), warmth (calor), pain (dolor), and swelling (tumor). As seen in this elbow joint from another patient:
Our patient has inflammatory arthritis. Next we think about the number of joints that are involved.
 There are 2 joints involved, putting us in the category of oligoarticular arthritis, which helps to focus our differential diagnosis. Now we can seek hypothesis-driven information from the history and the exam.
There are 2 joints involved, putting us in the category of oligoarticular arthritis, which helps to focus our differential diagnosis. Now we can seek hypothesis-driven information from the history and the exam.
 We were looking for nail bed pitting but instead find what looks like a drop of oil. Ah, the oil drop sign. So we perform a full skin examine and the following findings were hiding on the nape of the neck (had to lift up her hair to see it) and the back of her ear.
We were looking for nail bed pitting but instead find what looks like a drop of oil. Ah, the oil drop sign. So we perform a full skin examine and the following findings were hiding on the nape of the neck (had to lift up her hair to see it) and the back of her ear.
Patient 15: Hyperpigmentation


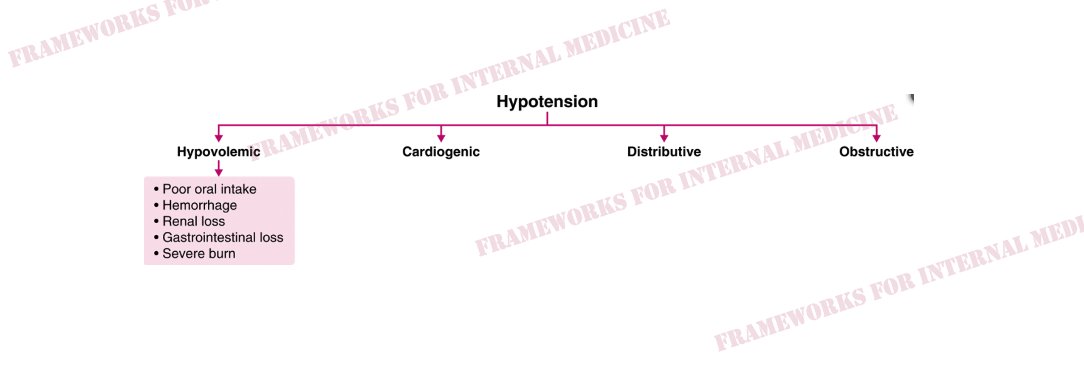
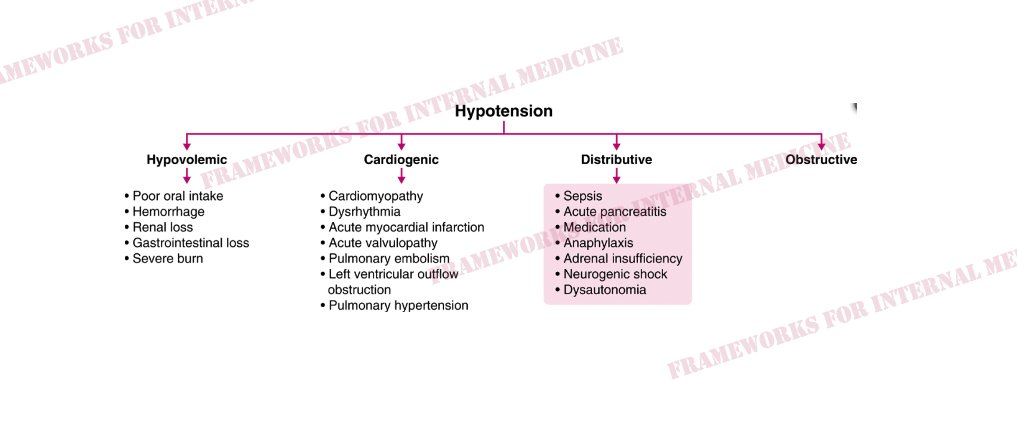
Let’s consider the hypotension. Physical examination is critical in determining which “category” of hypotension we are dealing with. How can physical exam help
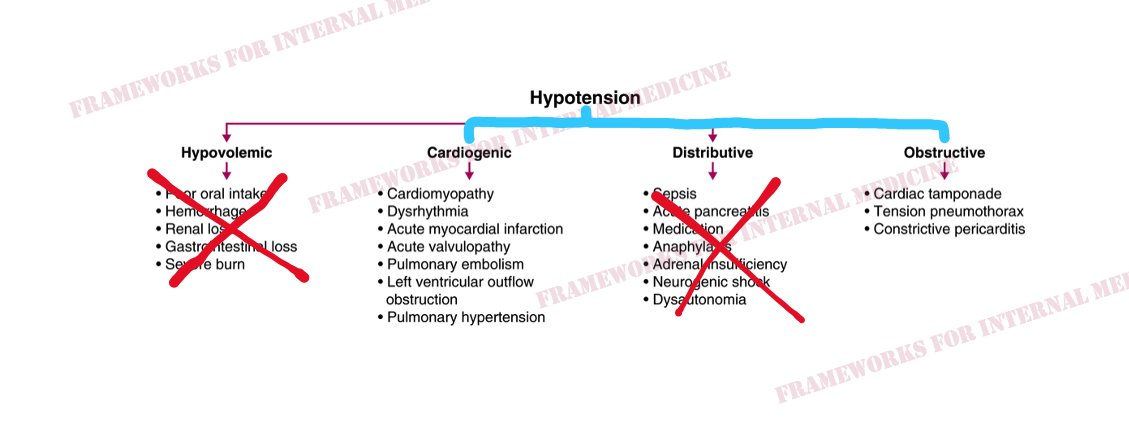
 In this case, JVP is low and extremities are warm.This pattern is characteristic of distributive hypotension. Do any of the conditions listed there match your initial hypothesis?
In this case, JVP is low and extremities are warm.This pattern is characteristic of distributive hypotension. Do any of the conditions listed there match your initial hypothesis?
 A confirmatory test is your next step. Serum cortisol is 4.4 ug/dL 60 minutes after a 250 ug injection of ACTH. (Normal adrenal function is established when serum cortisol level is >18 ug/dL after ACTH stimulation.)
A confirmatory test is your next step. Serum cortisol is 4.4 ug/dL 60 minutes after a 250 ug injection of ACTH. (Normal adrenal function is established when serum cortisol level is >18 ug/dL after ACTH stimulation.) You have now confirmed the diagnosis of adrenal insufficiency. The next question is, is it ACTH-dependent or ACTH-independent? A plasma ACTH level is necessary to make this determination. But based on your physical exam, you already know the answer.
You have now confirmed the diagnosis of adrenal insufficiency. The next question is, is it ACTH-dependent or ACTH-independent? A plasma ACTH level is necessary to make this determination. But based on your physical exam, you already know the answer.
 ACTH stimulates melanocortin-1 receptor in the skin –> hyperpigmentation. It usually occurs first in areas of skin under pressure, such as the knuckles or elbows, as in this case below. How long has she been feeling ill? The fingernails provide a clue.
ACTH stimulates melanocortin-1 receptor in the skin –> hyperpigmentation. It usually occurs first in areas of skin under pressure, such as the knuckles or elbows, as in this case below. How long has she been feeling ill? The fingernails provide a clue.
 Adrenal crisis is usually triggered by acute illness (in this case bacterial colitis). Many of the symptoms/signs can be mistakenly attributed to the acute illness that triggers the adrenal crisis, causing the diagnosis to be missed.
Adrenal crisis is usually triggered by acute illness (in this case bacterial colitis). Many of the symptoms/signs can be mistakenly attributed to the acute illness that triggers the adrenal crisis, causing the diagnosis to be missed.Patient 16: Chronic Diarrhea with heart problem
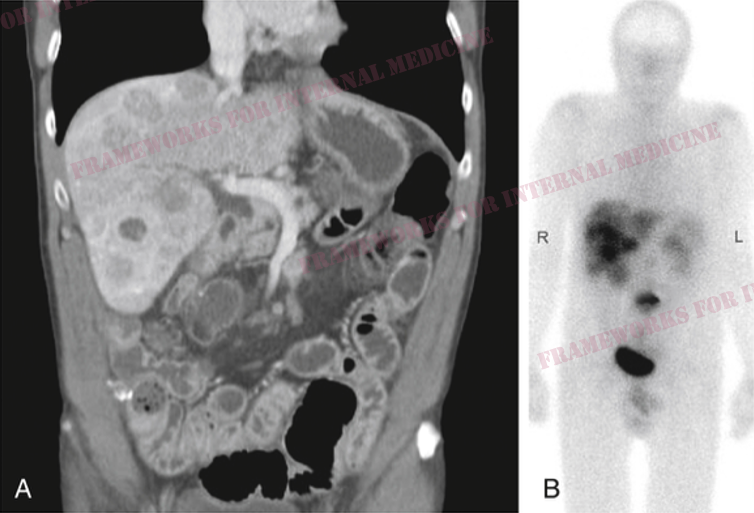
A 55 y/o man presents for evaluation of chronic diarrhea. We walk into the room to meet him. We have an opportunity to make an “augenblick” diagnosis – one that can be made in the blink of an eye.
We listen to his heart to help confirm our hypothesis (best with headphones). There is a holosystolic murmur over the LLSB. Notice that the intensity of the murmur seems to vary in a regular cycle? It gets louder/quieter/louder/quieter. What is the significance of this?
The augmentation of the murmur during inspiration is known as Carvallo’s sign, and indicates that the abnormal heart sound is coming from the R-side of the heart. Here is a more dramatic example in a different patient with tricuspid stenosis:
Patient 17: Dyspnea and high arches
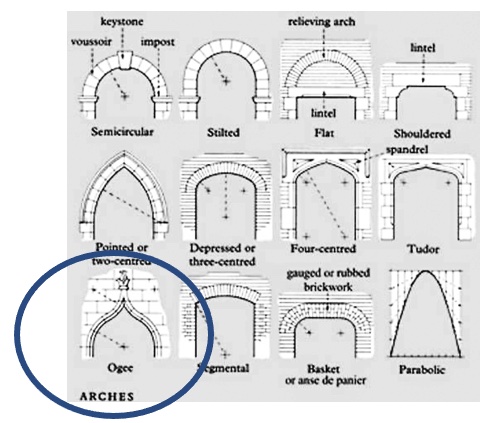
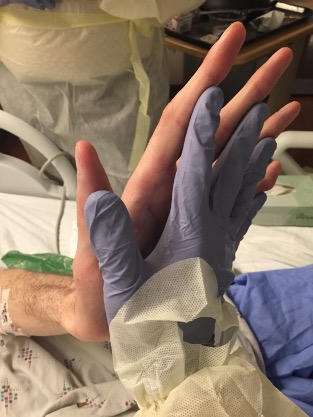
A young man presents with dyspnea. We start with his hands. My hand is gloved in the second photo (for frame of reference, I can palm a basketball). Our patient has a finding that should generate a hypothesis.
A high-arched palate. Otherwise note as an “ogival” arch. These arches are pointed at the top and are a key feature of Gothic architecture, beginning in the 12th century.

Now we must ask, how can Marfan’s syndrome lead to dyspnea? Before we auscultate, we anticipate what the heart will sound like. “The ears can’t hear what the mind doesn’t know.” Take a listen. (Best with headphones.)
Patient 18: Old man with a masked face
This patient presents for evaluation after family noticed he’s been “slowing down” recently. We walk in to meet him. How old do you think he is? Perhaps there is already a clue to the underlying diagnosis.
 After speaking with him, our clues are starting to add up.
After speaking with him, our clues are starting to add up.A classic finding is present. We move on to evaluate his gait, anticipating what we might see. And we see it
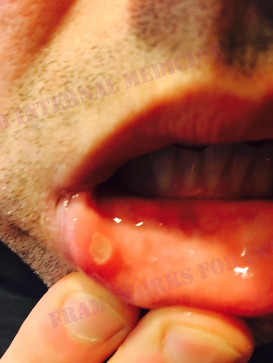
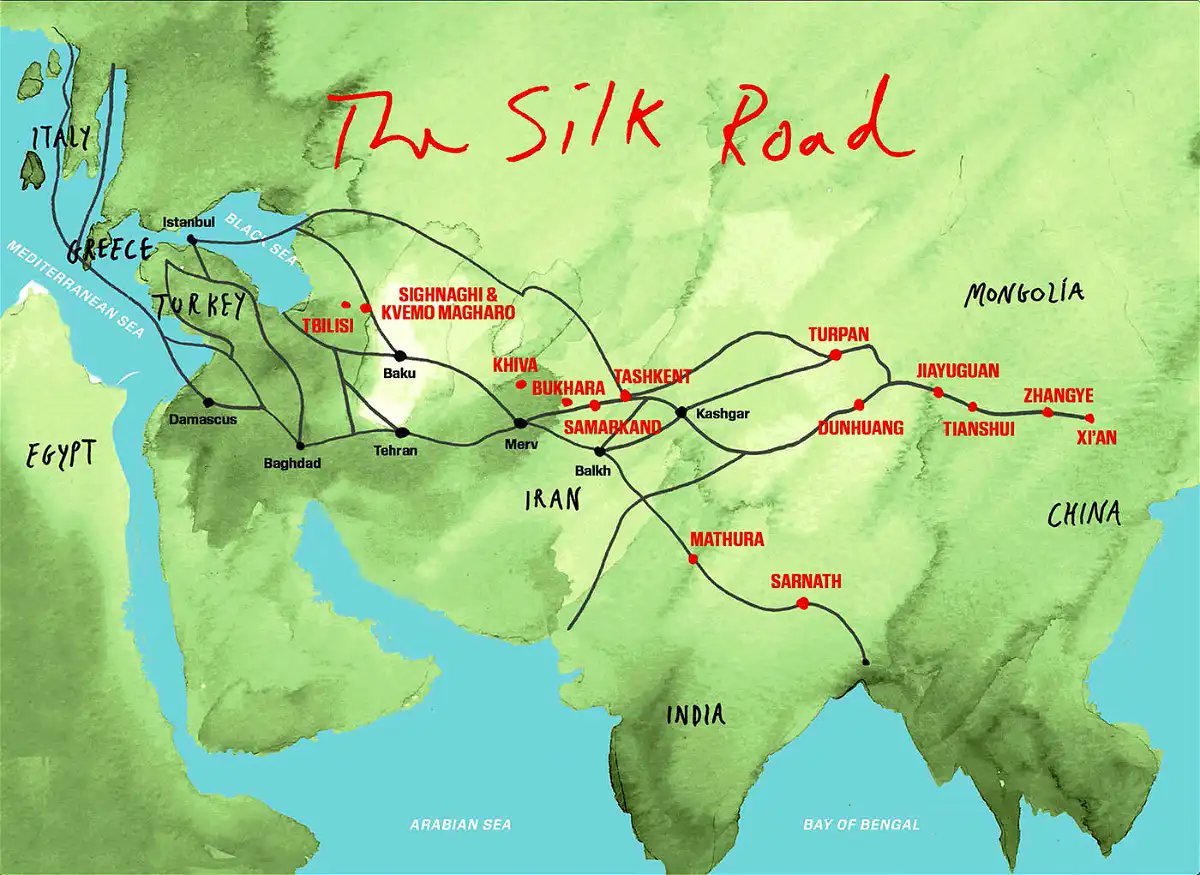
Patient 19: Chest pain, joint pain, erythematous nodules and the Silk Road.
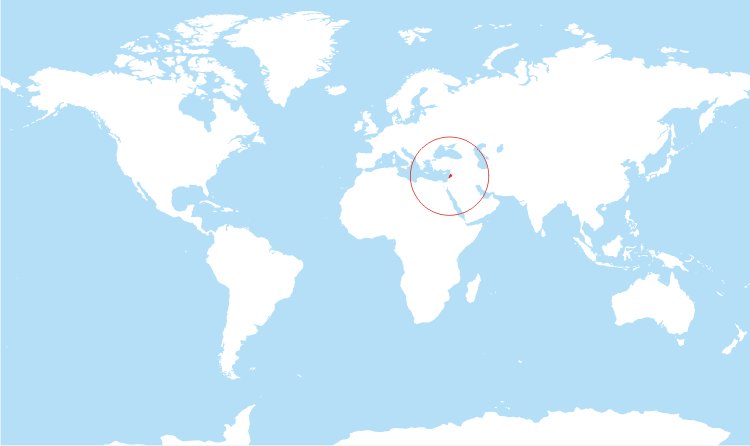
A young Lebanese man presents with several days of chest pain. Let’s remind ourselves where Lebanon is on the map. It may prove valuable “down the road”.
Now let’s deal with his chest pain. It can be helpful to think of chest pain as either cardiac or noncardiac in nature. The history and exam will point you down one “road” or the other
.
Our patient’s pain is substernal, sharp, and worsens when he breaths deeply. We auscult the heart with a hypothesis in mind, anticipating what we might hear. “The ears can’t hear what the mind doesn’t know”.
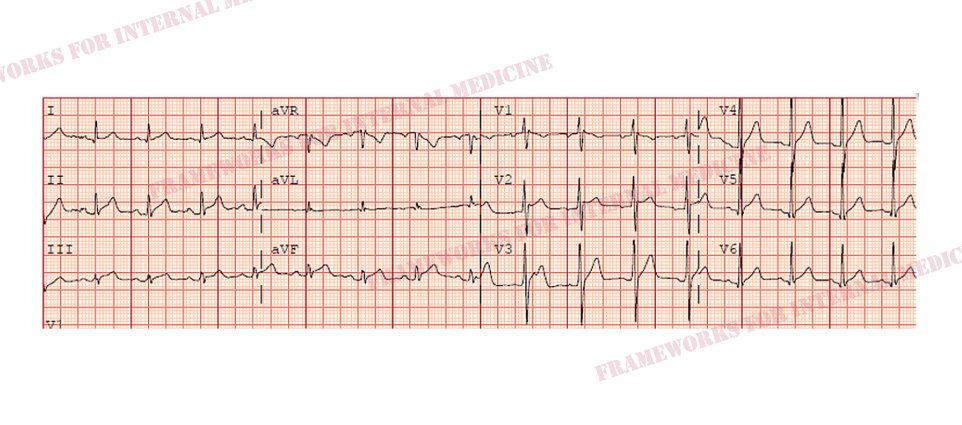
The quality of the pain does not sound like angina, but let’s look at his EKG to help us rule out ACS and confirm our hypothesis. We anticipate what we might see. “The eyes can’t see what the mind doesn’t know”.
Indeed, diffuse ST elevation, diffuse PR depression, and PR elevation in aVR are consistent with our hypothesis of acute pericarditis.
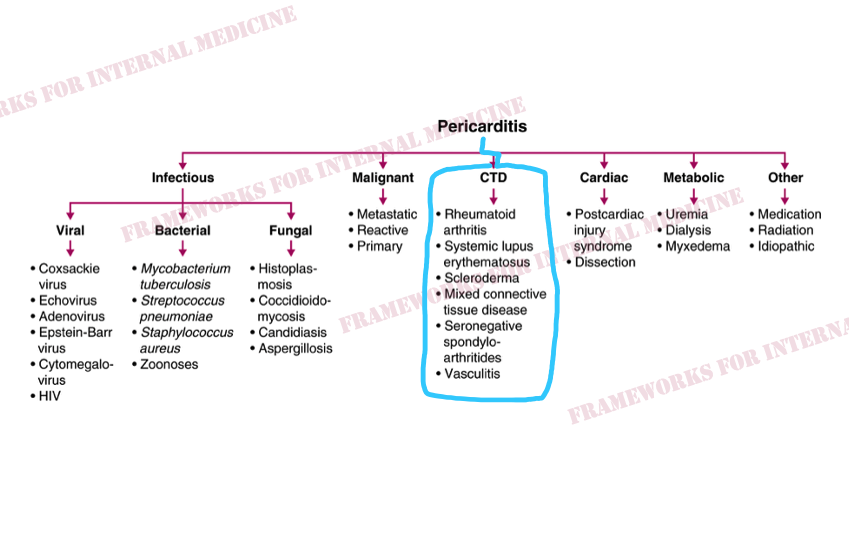
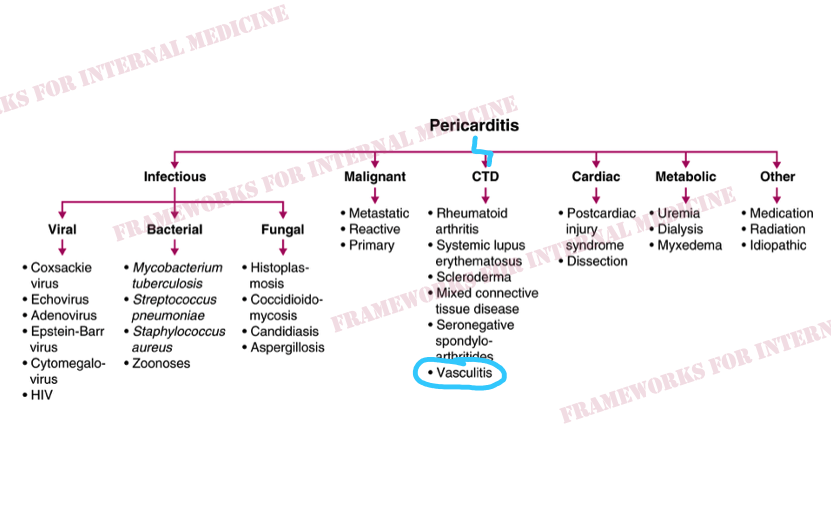
But why does our patient have pericarditis? Let’s review the main causes.
Additional hypothesis-driven history reveals joint pain and stiffness, especially in the morning. This points us toward a rheumatologic cause of pericarditis (connective tissue disease).
The skin and mucosa can be rich sources of clues to particular rheumatologic conditions. We start with the lower extremities and find these tender erythematous nodules on both shins.
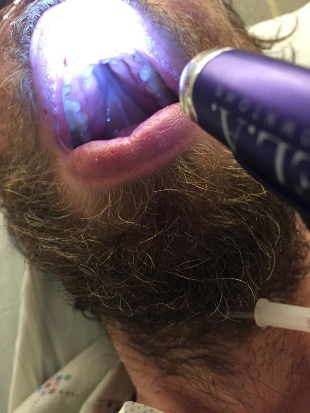
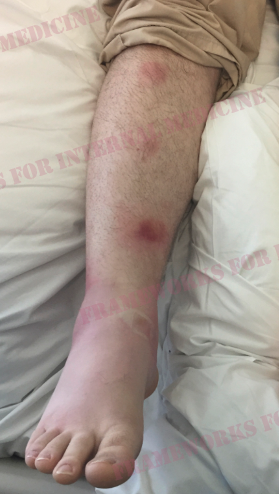
We ask the patient if he has any other painful spots on his body. He pulls down on his lower lip and shows us this painful lesion, which he says shows up from time to time in his mouth and on his scrotum and penis.
Let’s come back to Lebanon. It is a country in the Levant, with Syria to the north and east, Palestine to the south, and the Mediterranean Sea to the west.
The territory of modern-day Lebanon was part of an ancient network of trade routes, known as the Silk Road, which extended from eastern Asia to the Mediterranean.
Silk Road disease, AKA Behçet’s disease, is a form of systemic vasculitis. It is more common in peoples whose ancestors inhabited the lands around the ancient Silk Road, including Lebanon. The pericardium is the most common site of cardiac involvement in Behçet’s disease.
We diagnosed Behçet’s with our eyes and ears and a little help from geography and ancient history. Genetic ancestry – and its imperfect surrogate terms – can provide an important clue to diagnosis.
Patient 20: Dyspnea with Hypotension
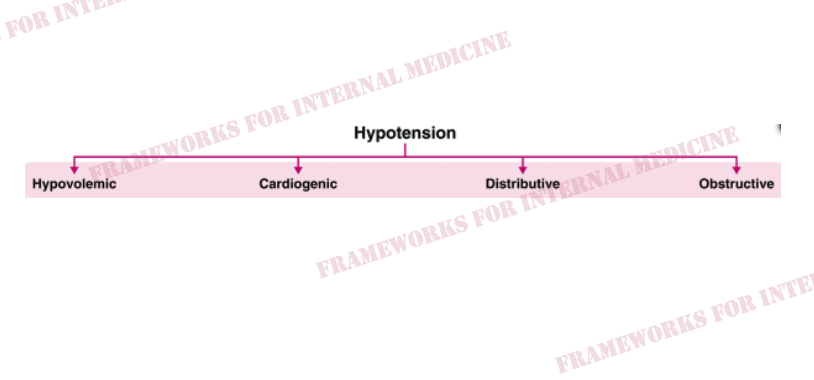
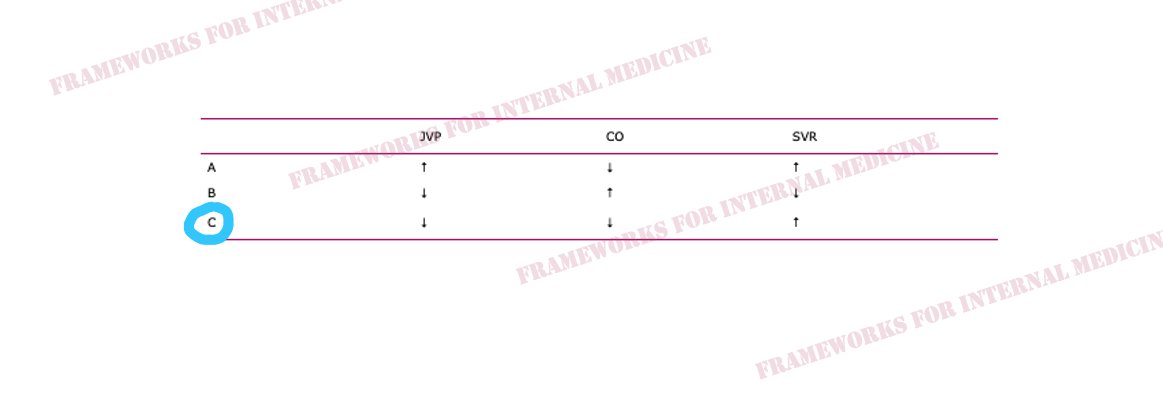
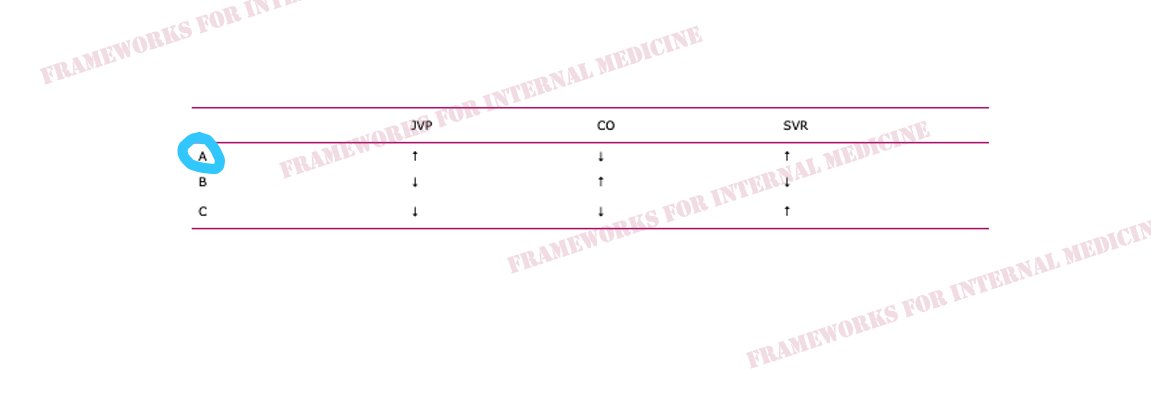
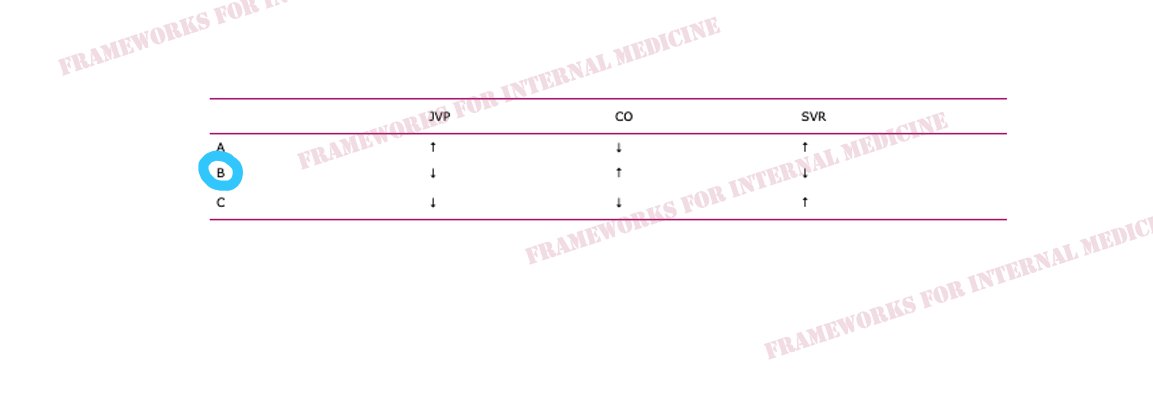
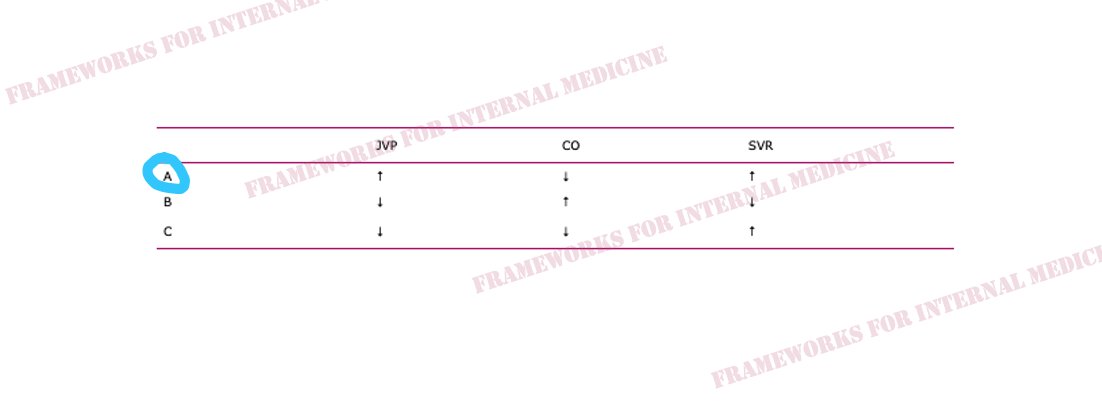
Hypovolemic hypotension occurs as a result of decreased hydrostatic pressure within blood vessels, and decreased cardiac preload with an associated decrease in cardiac output. It is characterized by decreased CVP (primary issue), decreased CO, and increased SVR (choice C).
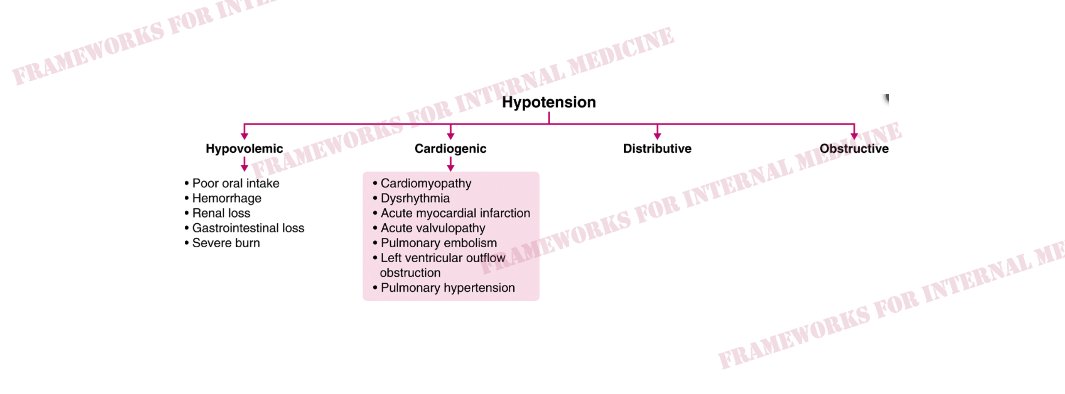
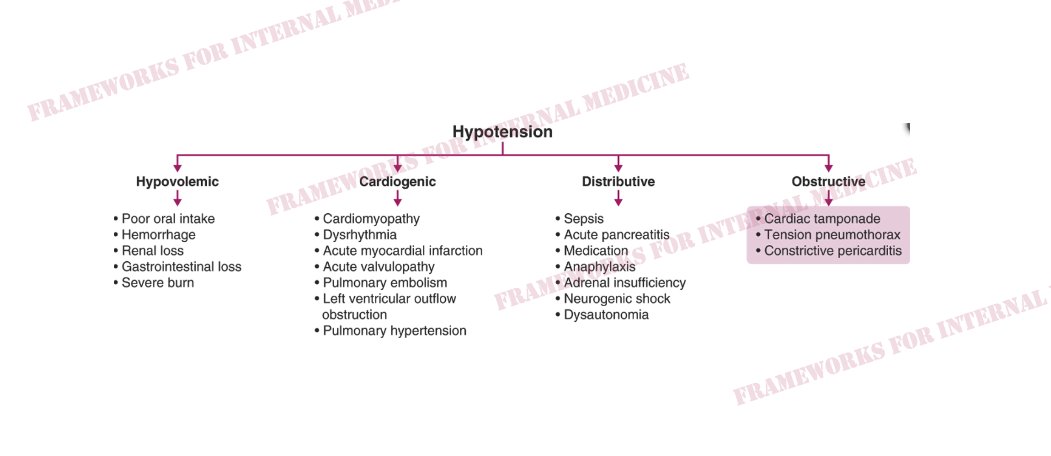
What are the causes of hypovolemic hypotension?
Cardiogenic hypotension occurs as a result of pump failure with an associated decrease in cardiac output. It is characterized by increased CVP, decreased CO (primary issue), and increased SVR (choice A).
What are the causes of cardiogenic hypotension?
Distributive hypotension occurs as a result of pathologic peripheral vasodilation with an associated decrease in SVR. It is characterized by decreased CVP, increased CO, and decreased SVR (primary issue) (choice B).
What are the causes of distributive hypotension?
Obstructive hypotension occurs as a result of decreased cardiac filling with an associated decrease in both preload and cardiac output. It is characterized by increased CVP (primary issue), decreased CO, and increased SVR (choice A).
What are the causes of obstructive hypotension?
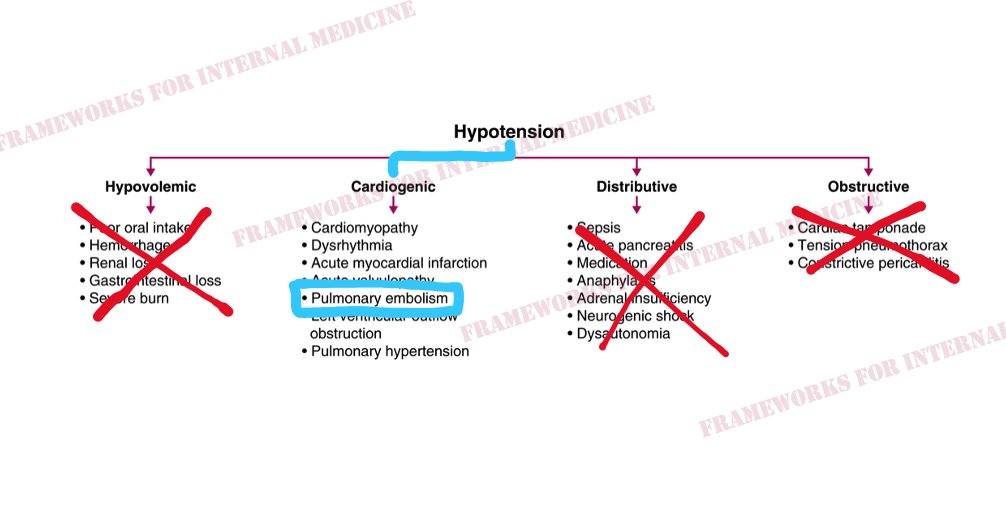
Back to our patient. His extremities are cool to the touch. What did you notice in the neck? JVP is elevated. This immediately cuts our differential diagnosis in half.
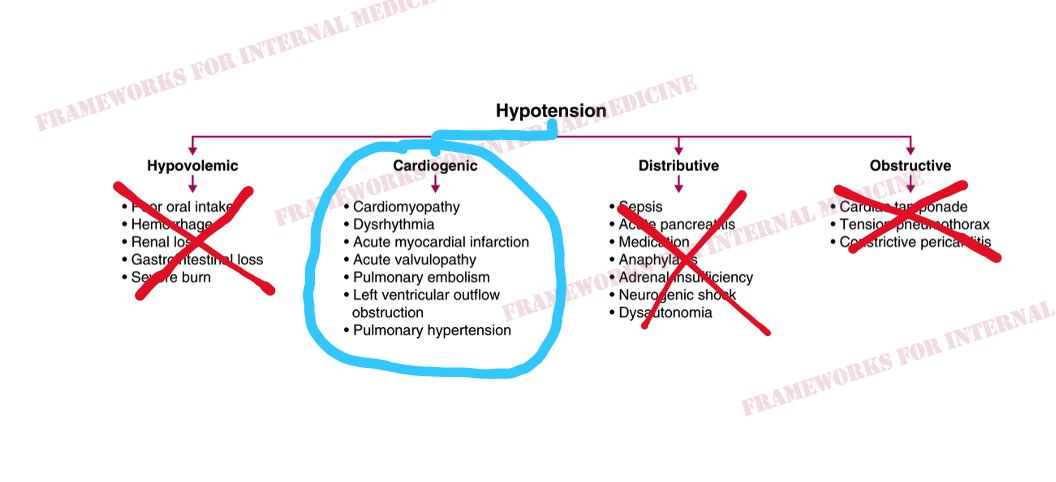
Is there a clue in the neck veins? You bet there is. Let’s look at the original video. Which component of the waveform stands out to you?
As we ponder the causes of sudden-onset RV failure, we begin to narrow our focus. And we anticipate what we might hear when we listen to the heart. (Sound up.) “The ears can’t hear what the mind doesn’t know.”
Patient 21: Chest Pain, Dyspnea
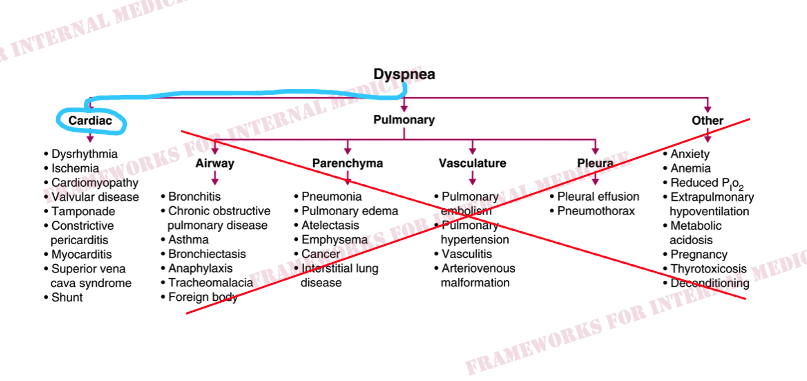
There is a 3-component pericardial friction rub. Telling us the patient has acute pericarditis. Sharp/deep X/Y descents in the venous waveform is known as the W sign, and in combination with Kussmaul’s sign, is indicative of pericardial constriction.

Patient 22: Hematuria with skin lesions
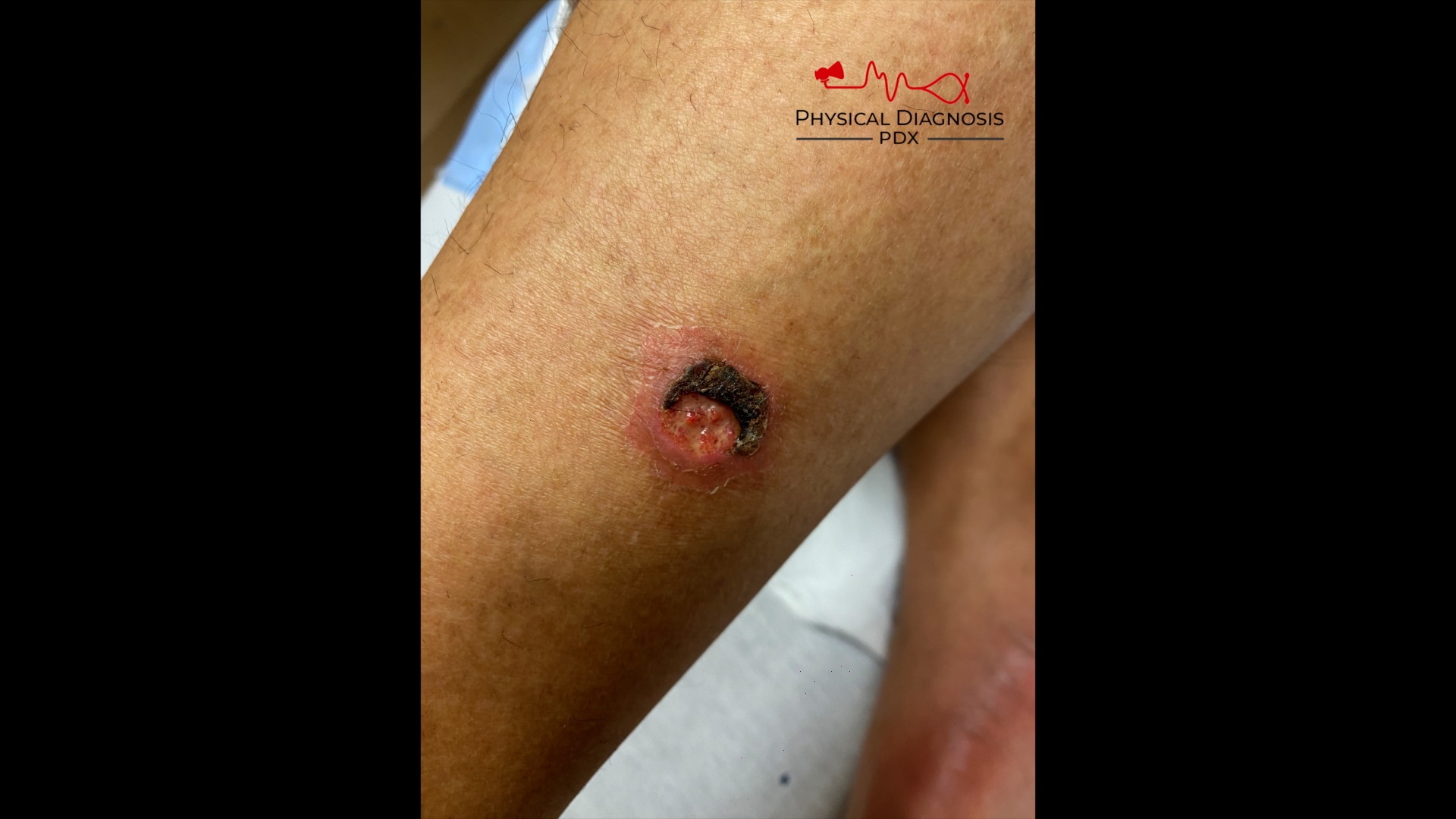
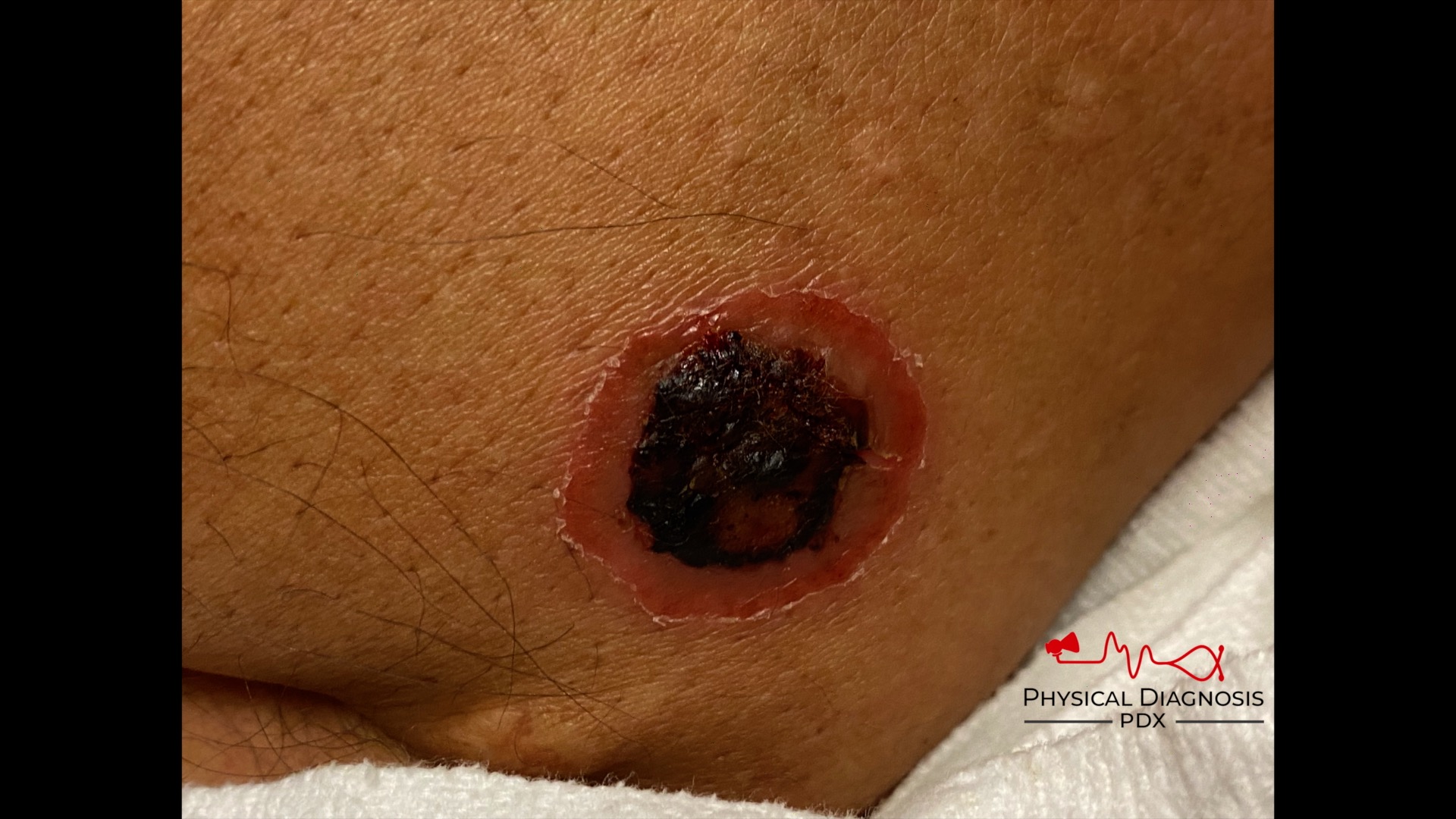
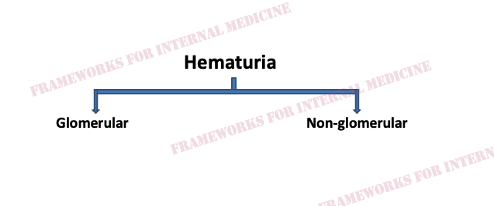
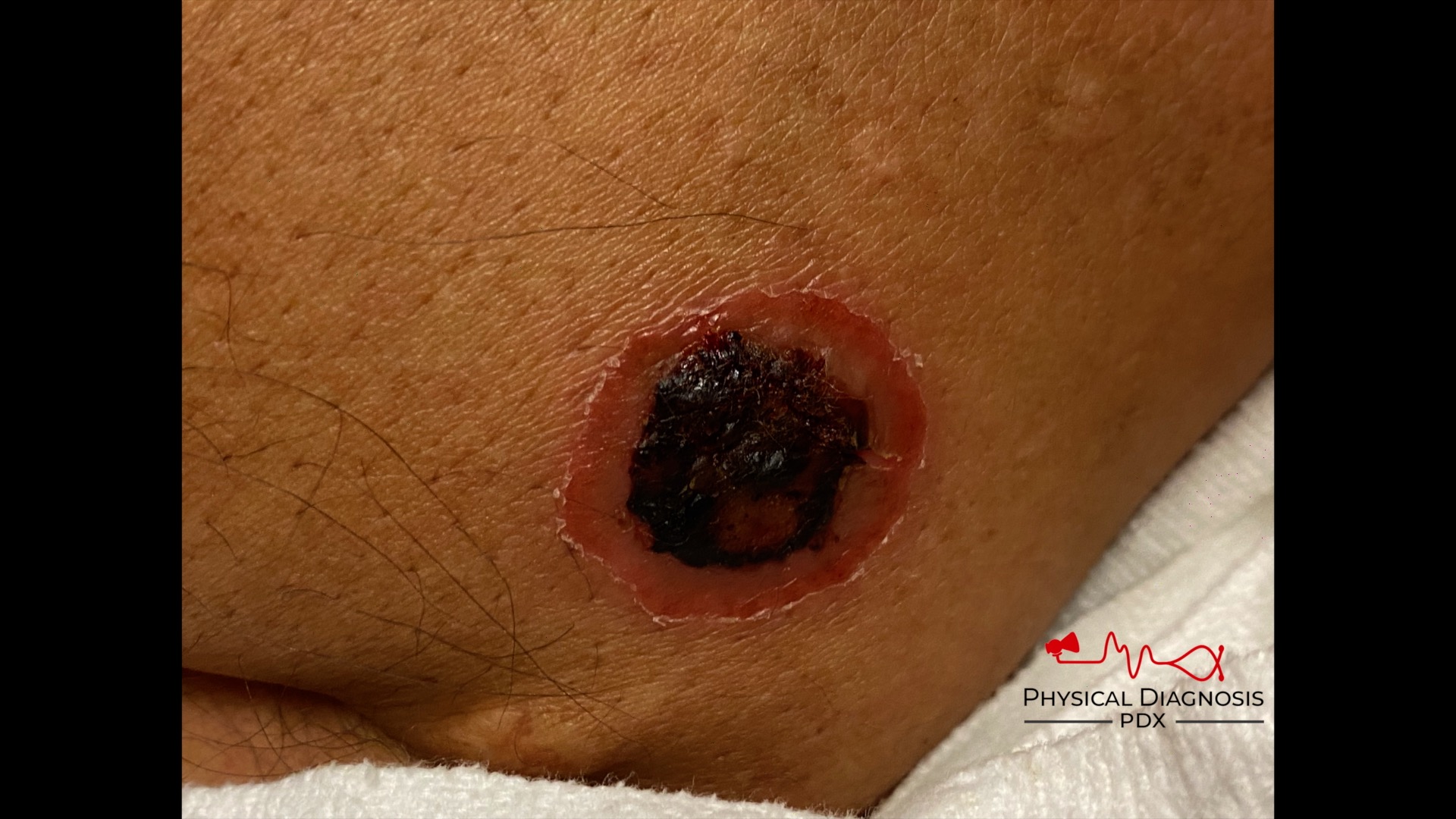
How can we tell if the bleeding is glomerular or not? We have to evaluate the urine sediment. But the eyes can’t see what the mind doesn’t know. So what are we looking for? (Images courtesy of
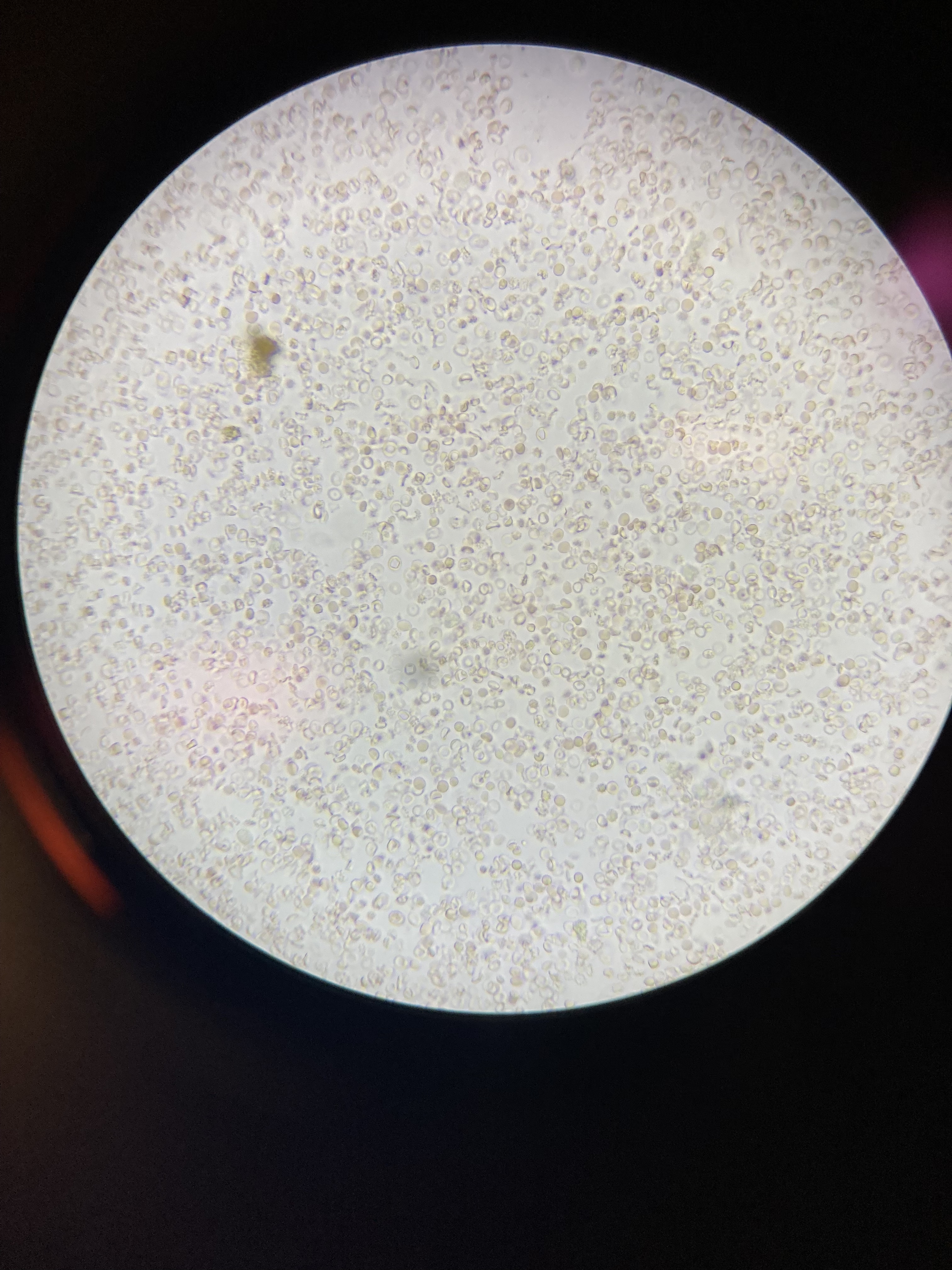

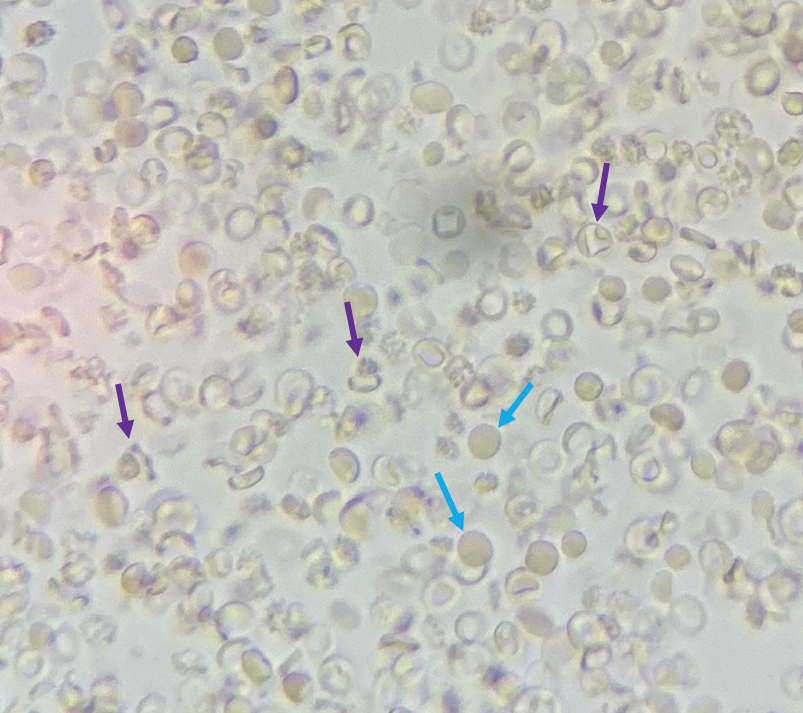
When those RBCs squeeze through the glomerulus they end up in the tubules, where they can form casts. Check out what
found below. That’s an RBC cast ! Another clue that our bleeding source is glomerular.
Patient 23: Nose and gum bleed with skin rash
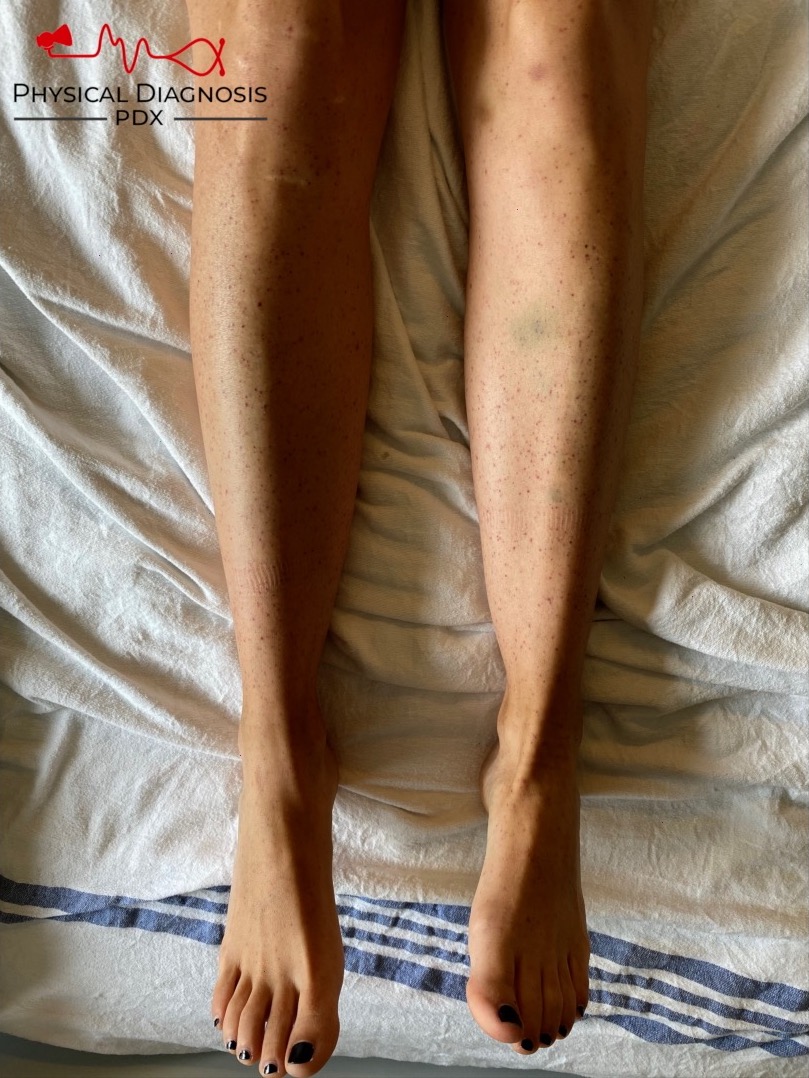
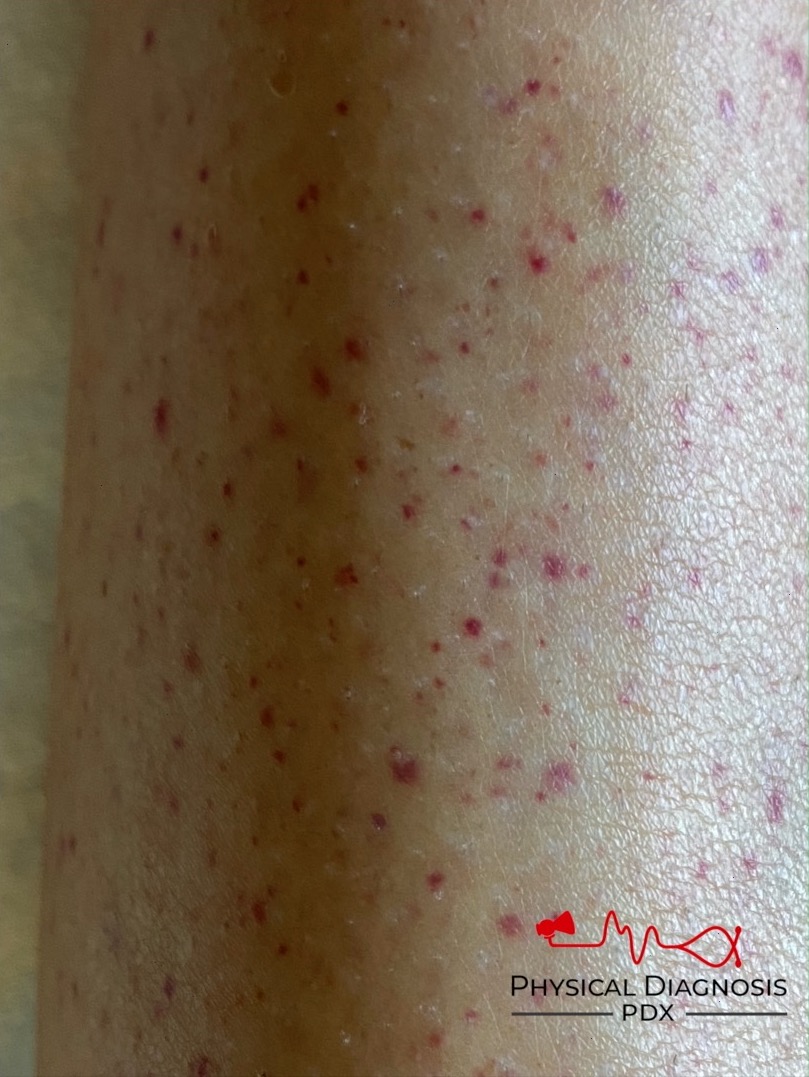
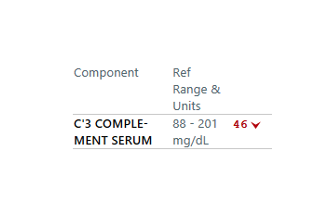
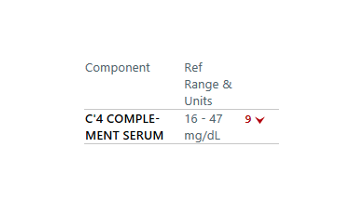
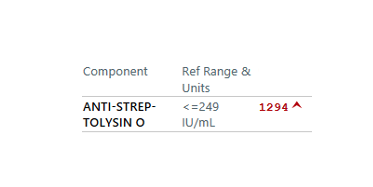
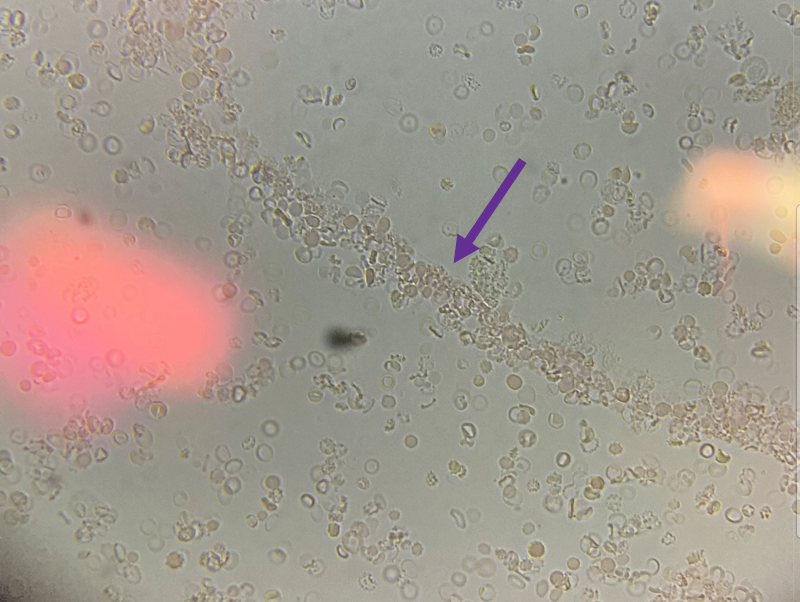
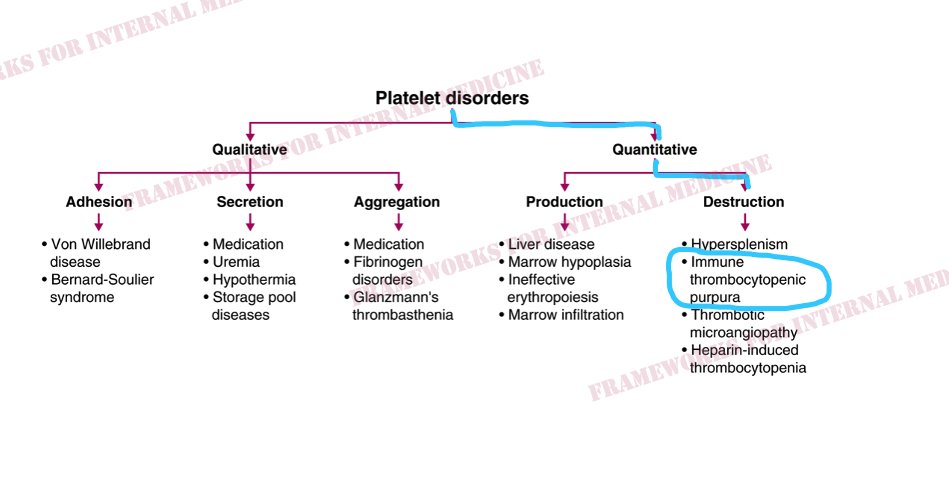
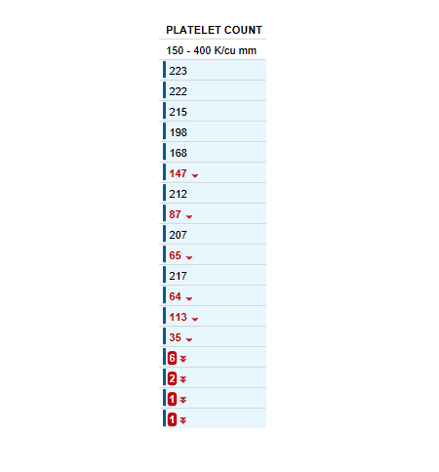
A platelet disorder could explain these symptoms and physical findings. But is the platelet issue qualitative (platelets are normal in number but abnormal in function) or quantitative (platelets are normal in function, but abnormal in number)?
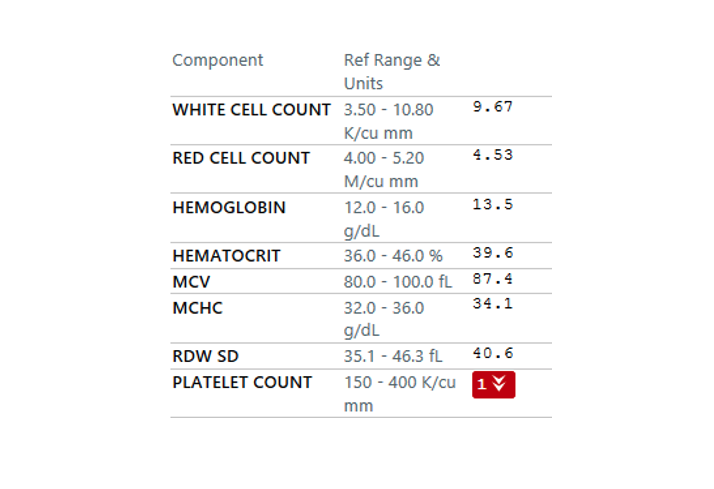
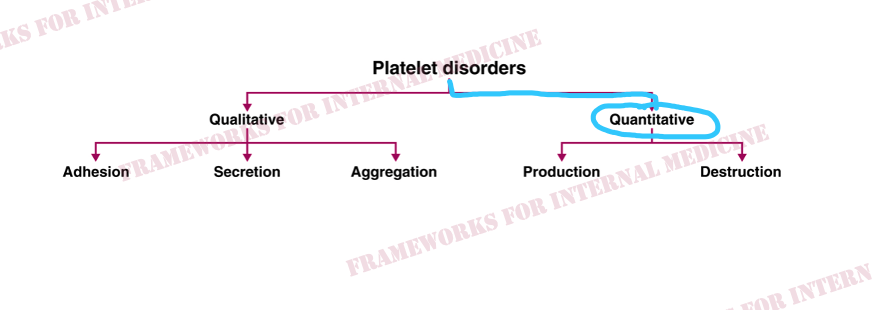
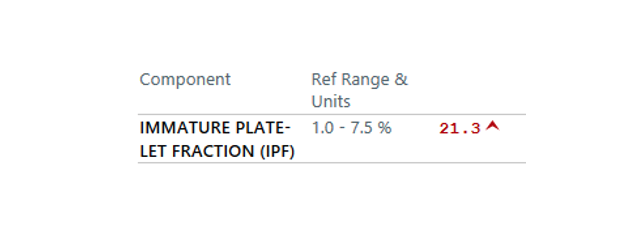
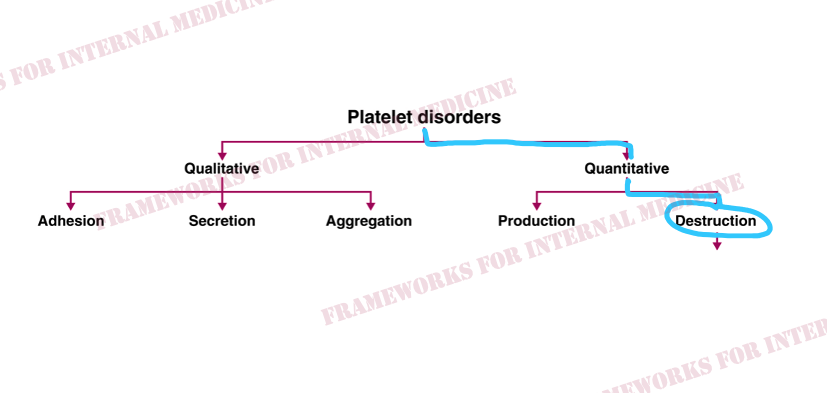
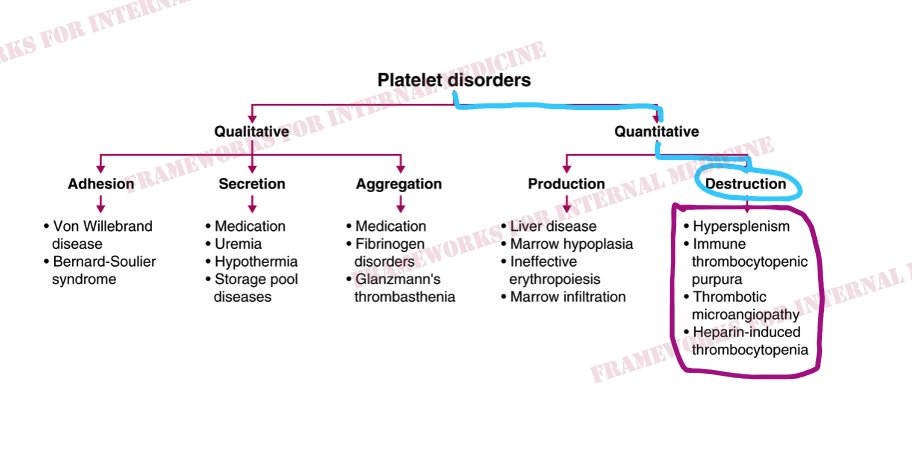
Patient 24: Dyspnea and Hypoxemia
A man undergoes biopsy and plate/screw placement for a pathologic hip fracture and returns to the floor with new dyspnea and hypoxemia. Hypoxemia can be intimidating. Unless you have a system. Let’s go through the case.

The first thing we want to know is the A-a gradient. The difference between partial pressure of oxygen in the alveolar space (A) and arterial space (a).

Here is our patient’s ABG on room air.

PAO2 = 0.21(713) – 17/0.8 = 129 mm Hg.
PaO2 = 53 mm Hg
A-a gradient = 129 – 53 = 76. It’s elevated.
This narrows our differential considerably. We know we’re not dealing with hypoventilation from anesthesia. And now we can focus on the causes of elevated A-a gradient.

Our patient has clear lungs on exam and on CXR.

The exam narrows our differential even further. Hypoxemia with elevated A-a gradient and clear would be incompatible with most causes of physiologic shunt and impaired diffusion.

We have a patient who is hypercoagulable from an underlying malignancy and surgery and the hypoxemia is sudden in onset. These historical features should generate a hypothesis that leads us to his neck exam. What finding is present? (Turn sound on.)
The elevated JVP and Kussmaul’s sign points to pulmonary embolism. We order an EKG, but while we wait we should begin to anticipate what electrocardiographic findings might be present in the setting of a PE.
The eyes can’t see what the mind doesn’t know.

Sinus tachycardia, new RBBB, R>S in V1, T wave inversions V1-3, and everyone’s favorite finding S1Q3T3. All indicative of right heart strain. Here is the preop EKG taken just 8 hours prior:

Hypothesis-driven history led to hypothesis-driven physical exam which led to hypothesis-driven EKG, which has now led to hypothesis-driven CTA:
And we have our diagnosis. (Either clot or fat emboli from orthopedic surgery.)

For me, it would be impossible to practice medicine without the history and physical examination. And I use frameworks to organize my differential and workup in a hypothesis-driven way.
Patient 25: Progressive Numbness and Paresthesias
A 35 y/o woman presents with numbness in feet and legs, paresthesias, imbalance, and frequent falls, progressing over a period of months.
Your astute med student notices high arched feet and bent toes. What do these findings suggest?

The history along with the presence of pes cavus (high arch) and hammertoes (toes bent at middle joint) suggest peripheral neuropathy (eg, polyneuropathy). Let’s perform a hypothesis-driven exam. What would we expect the reflexes to be like in a patient with polyneuropathy?
Our pt has brisk upper extremity reflexes and absent lower extremity reflexes. Polyneuropathy is usually length-dependent, beginning in the legs before affecting the arms.
Next, we’ll test sensation, starting with pain and temperature (small fiber, anterolateral cord).
Pain/temp deficits are present, but not severe. How about vibration and proprioception (large fiber, dorsal column)?
Quite abnormal.
How about Romberg? It evaluates proprioception (not cerebellum!). To maintain balance at least 2 of these must be intact: vision, vestibular function, proprioception. When proprioception is impaired, closing the eyes results in a loss of balance, seen here:
The vibration/proprioception deficits are more severe than the pain/temp deficits. And what about those brisk upper extremity reflexes?
Does this pattern of involvement suggest any particular etiology in our differential?

We can begin to narrow our differential almost immediately. Our pt has acquired this condition later in life, making hereditary causes unlikely. The absence of fever and other constitutional symptoms makes inflammatory polyneuropathy less likely (though still possible).

Combo of cord involvement + peripheral neuropathy is known as myeloneuropathy.
Corticospinal tract (brisk UE reflexes) and dorsal column involvement (vib/prop loss) = subacute combined degeneration (SCD).
This leads to a hypothesis-driven test:

Copper level is also low, which is likely contributing as well. Copper deficiency can present the same way as B12 deficiency, with myeloneuropathy/SCD.

Additional history reveals chronic heavy alcohol consumption and poor diet, risk factors for the development of vitamin B12 and other micronutrient deficiencies.

Patient 26: Dyspnea
A 70-year-old man presents with dyspnea. What do you notice when you first meet him?

This finding should generate a hypothesis, which we will circle back to eventually. Before we do, let’s talk about dyspnea. The two main systems responsible for dyspnea are the heart and the lungs.

The jugular venous pulse can serve as a pivot point. It can take you toward or away from the heart. With this in mind, let’s evaluate the patient’s neck. Here, he is in the upright position. (Sometimes the jugular venous pulse is better seen on the left.)
The JVP is elevated. We know we are dealing with something related to the heart.

More history reveals orthopnea and PND. Our patient has the clinical syndrome of heart failure. Systolic function is preserved on echo.

Let’s return now to our initial physical finding. Is there a cause of diastolic HF on that framework that is associated with easy bruising and “raccoon eyes”?

Our hypothesis leads us to order an EKG. What are we specifically looking for on the EKG?
“The eyes can’t see what the mind doesn’t know.”

The EKG demonstrates reduced limb lead QRS voltage (all limb leads <0.5 mV) with preserved precordial voltage, and P-wave prominence, characteristic of cardiac amyloidosis.

Cardiac amyloidosis was confirmed with endomyocardial biopsy.

Patient 27: Dysphagia
A 76 y/o man presents with swallowing difficulty. So why are we looking at his hands?

What’s your approach to dysphagia?
The first thing we want to determine is whether dysphagia is oropharyngeal or esophageal.

The patient not have trouble initiating a swallow and there is no choking, coughing, or drooling. Food material seems to get stuck in the middle of his chest.
These features point away from oropharyngeal dysphagia and toward esophageal dysphagia.

Next we want to use the history to determine if there is a structural or motility issue. The patient has had trouble swallowing both solids and liquids from the onset of symptoms.
This suggests a motility disorder.

Symptoms have been progressive over a period of months.
So are we dealing with achalasia or scleroderma?

Let’s take a closer look at the hands to help answer this question.
Evidence of digital ulcerations provides a clue.

You notice a small white nodule near the distal interphalangeal joint of the middle finger, seen below. What is this?

You also notice small erythematous lesions on the fingers. They blanch when compressed. What are these called?
History and physical exam lead you to the diagnosis of scleroderma (systemic sclerosis).

Patient 28: Elevated JVP
I was in the hospital with a group of students when a cardiologist spotted us. “Go see the patient in such and such room, you might like his exam.”
So we go in blind.
We notice that he’s young (30s). Next, we examine the neck. Here he is in the upright position.
JVP is elevated and Kussmaul’s sign is present. Normally the JVP goes down with inspiration.
In addition to Kussmaul’s sign, there is a second qualitative finding, better seen in this video. What part of the waveform catches your eye? Outward or inward?
The students start to name the things that could cause Kussmaul’s sign as well as giant a waves. The as-yet unknown diagnosis is among their hypotheses. Before we listen, we anticipate. The ears can’t hear what the mind doesn’t know.
Over the apex there is a split S1.
Next we hear a murmur over the left lower sternal border. Our collection of hypotheses allowed us to predict that this murmur might be present.
When determining the cause of a murmur, timing is key. Is it systolic or diastolic? This will cut our differential diagnosis in half.

Timing with the cardiac cycle allows us to determine that the murmur is occurring in diastole. It is also quite long in duration, which can serve as nice clue to a diastolic murmur (since diastole is longer than systole).

The next question we want to know is the shape of the murmur. Is it the classic decrescendo murmur of aortic regurgitation or does it get louder at the end?
Our patient’s murmur clearly crescendos at the end of diastole.

So are we dealing with mitral or tricuspid stenosis? How can we tell?
Did you appreciate that the murmur increases in intensity during inspiration? Carvallo’s sign tells us the lesion is on the right-side of the heart. Listen again.
Tricuspid stenosis explains all of our findings:
Kussmaul’s sign – RV can’t fill because of TS
Giant a wave – Atrial kick against a stenotic valve
Murmur – Atrial kick causes a crescendo diastolic murmur
Split S1 – TS causes delayed closure of tricuspid valve

Our patient has TS as a result of a bioprosthetic valve (received after a bout of native valve IE) that is functionally “too small.”
Patient 29: Weakness
A man presents with weakness. Let’s walk through an approach to this problem.

The etiologies of weakness can be subdivided into 4 main categories:

What are the signs of an UMN lesion?
No (or minimal) muscle atrophy, no fasciculations, increased tone, + Babinski’s, and increased reflexes, the latter of which is demonstrated below in a different patient with a L-sided stroke.
What are the signs of a LMN lesion?
Severe atrophy, decreased tone, decreased reflexes, absent Babinski’s, and the presence of fasciculations, the latter of which is demonstrated below in a different patient with Guillain-Barré syndrome.
What is the hallmark clinical sign of the most common neuromuscular junction disorder?
Fatiguability, as demonstrated below in a different patient with myasthenia gravis. Notice that the ptosis significantly worsens after the patient stares at the ceiling for a minute.
Our patient has none of these clinical features, making a lesion of the UMN, LMN, and neuromuscular junction unlikely.

Does he have a myopathy? Let’s revisit the photo from the initial post. What finding is present?

Do you have a hypothesis? What other signs would you look for next?


Yep, he’s got those too.
We have just diagnosed dermatomyositis based on our physical exam and a little help from Frameworks.
Patient 30: Hyperglycemia
A 33 y/o F with carpal tunnel syndrome presents with polyuria and polydipsia. She has a fasting serum glucose of 212 mg/dL and a hemoglobin a1c of 9.7%.
Do you have an approach to hyperglycemia?

The first step is to determine whether we are dealing with insulin-dependent hyperglycemia or insulin-independent hyperglycemia.

Insulin-dependent hyperglycemia occurs as a result of insulin deficiency; insulin-independent hyperglycemia occurs despite the presence of insulin and is primarily the result of insulin resistance.

This can usually be determined clinically. When in doubt, obtaining simultaneous fasting plasma levels of glucose, insulin, and C-peptide can be informative. In the setting of hyperglycemia, insulin and C-peptide levels should be elevated; inappropriately low values are consistent with insulin deficiency (insulin-dependent hyperglycemia).
In this case, insulin and C-peptide are elevated.

It would be easy to call this type 2 DM and move on. The advanced clinician must consider secondary causes of insulin-dependent hyperglycemia. That’s why frameworks are so useful, even for a seemingly “every day” problems like hyperglycemia. The framework compels us to consider other possibilities.
Let’s revisit the photo at the start of the case.

What do you notice about the patient’s facial features compared to the driver license photo (taken 4 years prior)? Prominent facial features, most pronounced in the forehead (frontal bossing), nose, and lips, should generate a hypothesis.
With this hypothesis in mind you reach out and shake our patient’s hand. It feels moist and doughy. Here are the hands of a different patient with the same condition (courtesy @petesullivan):

With our hypothesis becoming more likely, we obtain retrospective history. The patient’s shoe size has increased over the years to the point where she can only fit into men’s shoes. Her ring size has doubled. She says her tongue has gotten larger and is difficult to move in her mouth.
We have all but made our diagnosis. Elevated serum levels of IGF-1 would be confirmatory.

Acromegaly is a rare condition that is caused by hypersecretion of GH and IGF-1, usually from a pituitary adenoma. Manifestations develop slowly over a period of years and vary depending on the levels of GH and IGF-1, patient age, tumor size, and the delay in diagnosis. Common features include headache, visual field deficits, acral and soft tissue overgrowth, macroglossia, macrognathia, arthralgias and arthritis, HTN, DM, sleep apnea, and carpal tunnel syndrome.
Viewing old photographs of a patient (eg, from a driver license) can be helpful in recognizing altered physical features caused by an underlying insidious disorder like acromegaly.
They will tell you physical exam doesn’t matter. They will also diagnose this patient with DM2. Don’t be like them.
Patient 31: Hemiplegia
A 50 y/o M presented with L hemiplegia, facial droop, and right gaze preference.
So why are we looking at his chest?
Let’s start with our approach to stroke. The first question we want to ask is whether it is hemorrhagic or ischemic.

Imaging is usually necessary to determine this. It reveals acute ischemic infarction of the R caudate, basal ganglia, and R anterior frontal and temporal lobes.

Flank pain prompted imaging of the abdomen which demonstrated a renal infarction.
In contrast to thrombotic strokes, which typically present in a single vascular territory, embolic strokes can involve multiple cerebral vascular territories, as well as non-cerebral vascular territories (eg, kidney).

So let’s get back to the precordial movement. There is an outward apical impulse and just to the left a retraction. The classic “see-saw” movement of a regurgitant valvular lesion.
Let’s take a listen to our patient’s heart, over the apex.
Classic holosystolic murmur of mitral regurgitation. In fact, it’s a 5/6. There is a palpable thrill and the murmur can still be heard with half the scope lifted off the chest. Echo confirms what we already know. Severe MR. And with a 1×1 cm vegetation.
Let’s review an approach to endocarditis.
Our patient does not have any systemic inflammatory conditions and the associated fever suggests infective endocarditis.

But the blood cultures have all been negative.

What is culture-negative endocarditis?

Our patient’s Bartonella test results:



A patient presented with stroke and we reasoned our way to a cardioembolic source (culture-negative Bartonella IE) using logic, physical examination, and diagnostic frameworks.













Very helpful. Thank u